What Are the Five States of Matter?
There are five states of matter that occur when physical forces are applied to them. These states are fairly easy to understand when their physical properties are observed. Depending on the type of matter that is being dealt with, the state of the matter may be more difficult to reach when applying these physical stressors. The best way to understand the state of matter is to choose matter that reaches the state the easiest and observe it.
Bose-Einstein Condensates as a State of Matter
The Bose-Einstein Condensate is a very special phase of matter. It is a very slow moving and unexcited atom that actually clumps together with other atoms creating super atoms. This only occurs at a temperature that is a few billionths of a degree above absolute zero. Although Bose and Einstein were not alive when it was truly discovered in 1995, Bose-Einstein condensates were named after them because they thought of it first. The only reason they could not prove it was because the technology of that time was incapable of producing the results.
Rubidium was the first observed Bose-Einstein Condensate. A large amount of energy is actually required to produce this low energy state of matter. The only way to create them now is to use lasers to attack the atoms and cool them. This is possible because bouncing photons off of matter requires more energy than it takes to fire the photons. This transfer of energy eventually super-cools the elements and creates Bose-Einstein Condensates. This state of matter is capable of grouping millions of atoms within the same physical space of a single atom.
Solids as a State of Matter
The solid is one of the states of matter that people learn about as children. Solids are dense states of matter that group large amounts of matter close together. This is what happens when water is frozen. The atoms that make ice from the water are arranged and packed more closely together. Solids are everywhere in our universe and are made from the simple atoms that have been around ever since the universe started. Solids can become liquid through the addition of energy such as heat. Some solids can become gas through a process called sublimation. The following are all examples of solid matter:
- Ice Cubes
- Metal Coins
- Sand
- A Computer Mouse
- Stones
Liquids as a State of Matter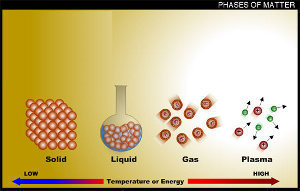
Most liquid matter that people come in contact with are very fluid in motion. Some liquids are thick while others are loosely compacted. This is called viscosity. Liquids can be really slow moving like putty or really fast moving like water. Depending on whether gravity is acting on the liquid, the liquid usually fills a flat surface container in the container’s shape. Liquids in zero gravity either turn into gasses or form a sphere into itself, gaining its own gravitational field. Liquids are an in-between state of matter that can become solid with compression (closer atoms) and gaseous with expansion (further atoms). The following are all examples of liquid matter:
- Water
- Blood
- Elemental Mercury
- Milk
- Oils
Gas as a State of Matter
Gas is an amazing state of matter. It can fill any container and exists unbound under many conditions. Gases are loosely packed atoms that are grouped randomly. They have moderately high energy and bounce around with ease. The cooling or compression of the atoms within a gas causes the gas atoms to get closer together. This can lead to the creation of liquids with the appropriate amount of pressure or loss of energy. Rapidly releasing the pressure in highly compressed gases can actually create solids without going through the liquid state phase. The following are all examples of gaseous matter:
- Water Vapor
- Carbon Dioxide
- Breath
- Nitrogen
- Helium
Plasma as a State of Matter
Seeing naturally occurring plasma is very rare on planet earth. Plasma is extremely high energy matter that is actually free electrons and ions of elements that bounce around at high speeds. Plasma is unique and does not occur often unless artificially created. Plasma’s high energy can make simple eerie glows or create heat that can melt through any physical element like a hot knife through butter. Plasma occurs naturally, but it is hard to get close enough to it to observe unless the observer knows where to look. Artificially created plasma is easier to observe but is not as spectacular as naturally occurring plasma. The following are examples of plasma matter that are observable:
- Star Energy
- Fluorescent Electrified Mercury Vapor
- Glowing Electrified Neon
- Lightning
- Electric Arcs
Mixtures of Matter
Matter has the ability to mix with other states of matter. This is possible because of solutes and solvents. Solutes are forms of matter that get dissolved and solvents are the forms of matter that dissolve the solute. This process can occur in a number of ways that usually involve physical stressors. Compression, turbulence, heating, and unstable states of matter can cause various mixtures to occur. The following are all examples of different mixtures that are possible with different types of matter:
- Compounds (Mixtures of different elements to create a single matter like concrete or plaster)
- Alloys (Mixtures of heavy solid elements like metal to make a new one like bronze, which is copper and tin or steel, which is iron and carbon/tungsten/chromium/manganese)
- Amalgams (Mixture of solid metal elements and liquid or unstable metal elements like quicksilver made from mercury and silver)
- Emulsions (Mixtures of matter that readily separate like water and oil or air and soap in foam)
- Solutions (Mixtures of matter that can be separated through a chemical process like salt water)


Comments - 5 Responses to “What Are the Five States of Matter?”
Sorry but comments are closed at this time.