Beta Particle
A beta particle is a form of ionizing radiation. Beta particles are closely related to other types of radiation such as gamma rays and alpha particles. In more scientific terms, a beta particle is a high-speed electron (and sometimes positron) that has been released from a degenerative radioactive nucleus. Beta particles are low mass and medium-energy and are one of the least-damaging types of radiation. Beta particles do, however, pose a large health risk. They contain the following: carbon-14, tritium, technetium-99, potassium-40, and strontium-90.
What Are Beta Particles?
Beta particles have a higher energy level than the associated electrons that orbit a nucleus. Beta particles are not radioactive. However, they can cause significant damage to humans as they can break chemical and create ions, which causes tissue damage. Beta particles are emitted when there is an excessive amount of neutrons in the atomic nucleus. When the amount of protons outnumbers the neutrons in a single nucleus, the neutrons turn into protons and electrons and are then pushed out from the nucleus at a high rate of speed. After this occurs, the atom’s stability and atomic number increase and becomes a new type of atom.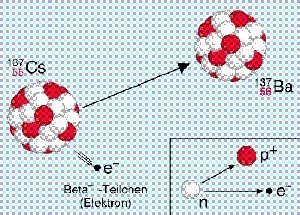
When Were Beta Particles First Discovered?
The first person to discover beta particles was Henri Becquerel in 1900. He demonstrated that beta particles were the same tying as electrons, which was not the common thought.
How Are Beta Particles Used?
Beta particles are used today to power space probes, lighthouses (in Russia), and in radioisotope thermoelectric generators. Russia’s use of the particles concerns many because the strontium contained in the associated lighthouses is more than the amount contained in the Chernobyl fire. Beta particles are also used in glow in the dark paint and radiotracers. Tritium and phosphorus-32 are used to achieve the desired effect in these cases.


Comments - No Responses to “Beta Particle”
Sorry but comments are closed at this time.