The Krebs cycle is named after German Biochemist Hans Adolf Krebs. The Krebs cycle refers to the variety of chemical reactions that happen within animal cells. Humans and animals utilize the Krebs cycle to process oxygen via respiration. The Krebs cycle also produces two byproducts: Carbon Dioxide (CO2) – Used oxygen atoms bind with carbon atoms and are exhaled as CO2 which is a waste byproduct. Adenosine Triposphate (ATP) – ATP is a high energy compound that is used in our protein synthesis process. With ATP, animals can create protein Read More
What Are Ionic Compounds?
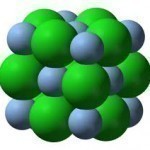
An ionic compound is any compound in which an electrostatic force holds the ions together. Ionic compounds are made of positively charged particles that rest against negatively charged particles in order to balance out the two particles’ magnetic imbalance. They can be found in both solid and liquid states, although solid ionic compounds are poor conductors. Ionic compounds can be used in a number of applications, most of which involve electrical conductivity. How Ionic Compounds Work All ionic compounds are made of microscopic crystalline structures composed of positively charged particles Read More
Methyl Iodide
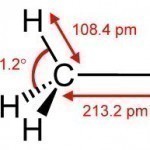
Methyl Iodide is a chemical compound that is relatively volatile. It is commonly found in the form of a dense, colorless liquid. It is also known as iodomethane and has the chemical formula CH3I (one carbon atom with three hydrogen atoms and a single iodine atom connected to it). Methyl iodide is a naturally occurring chemical compound that rice plantations emit in small amounts. Methyl Iodide Properties Methyl Iodide is a colorless liquid that turns purple when exposed to light for a long period of time because it is an Read More
Auger Electron Spectroscopy
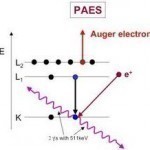
Pierre Auger discovered the Auger effect with x-rays that were involved in simultaneous cloud chamber usage. Auger observed that excited atoms emitted energetic electrons when the atoms relaxed. When the Auger effect was first discovered, it served little to the scientific community and was not utilized until the 1950s. The effect was then used to help improve the experimentation of chemical/compositional surface environments, in metallurgy, and in microelectronics. How Auger Electron Spectroscopy Works Auger Electron Spectroscopy is when specialized equipment that can test samples of materials is used to measure Read More
Potassium Carbonate Dihydrate

Potassium carbonate dihydrate (K2CO3.2H2O) is a white chemical. Salt from potassium carbonate and water from the dihydrate are mixed into a deliquescent (wet salt). It is a strong alkaline solution that garners a variety of uses. The salt tastes like salt and alkaline material. Potassium carbonate dihydrate is generally used as a nutritional supplement in livestock feed. It has several other uses when the water molecules are removed from the potassium carbonate dihydrate. When more water is added to potassium carbonate dihydrate, it dissolves into a solution. In its near Read More
What is an Acetylene Torch?
Acetylene is a hydrocarbon compound and is the simplest form of alkyne. Acetylene is colorless and extremely unstable in its pure form, which is why it is almost always mixed with other chemicals and handled as a solution. Acetylene was once the preferred gas in welding/cutting applications due to its ability to reach extremely high temperatures (3300 degrees Celsius/6000 degrees Fahrenheit) when mixed with oxygen. Acetylene produces the third hottest natural chemical flame, after cyanogen (4525 degrees Celsius) and dicyanoacetylene (4990 degrees Celsius). What is an Acetylene Torch? An acetylene Read More
Galvanic Corrosion

Galvanic corrosion occurs when two dissimilar alloys or metals come in contact with each other in a corrosive, conducting environment. When they touch, the anode metal corrodes faster than it would when submersed in the solution and the cathode metal corrodes much slower. Galvanic corrosion is not considered a bad quality, however, as the concept is commonly used in industry to protect more expensive metals by allowing less expensive ones to serve as anodes and be worn down instead. Why does Galvanic Corrosion Occur? When an electrolyte exists between two Read More
Potassium Gluconate

Potassium is a mineral that is found naturally in many foods. It is one of the most important minerals as it helps the body to carry out many different functions, the most important being its use in the beating of the human heart. In some cases, it is difficult for people to get enough potassium in their body. For example, there are some diseases that result in low potassium levels. If an individual has been vomiting for some time, his/her potassium levels may decrease. There are also some medicines that Read More
Limonene
One of the primary components of the oil that is extracted from the rind of citrus fruits is Limonene. When the juice is removed from a citrus fruit, the oil is also extracted from the rind, separate from the juice. The oil is then distilled so that flavor and other fragrance compounds can be removed from it. After this process is complete, the left over oil is called limonene or food-grade limonene. After the juicing process is completed, the peels are placed in steam extractors in order to harvest additional Read More
Vulcanized Rubber

Vulcanized rubber is a material that undergoes a chemical process known as vulcanization. This process involves mixing natural rubber with additives such as sulfur and other curatives. Vulcanization makes rubber much stronger, more flexible, and more resistant to heat and other environmental conditions. Vulcanized rubber makes both soft and hard objects, ranging from rubber bands to bowling balls. In fact, almost any rubber object consists of vulcanized rubber. How Vulcanized Rubber is Made It is necessary to use vulcanization to make commercial-grade rubber because natural rubber is not stable enough Read More


Share on: