What is a Nonpolar Covalent Bond?
Covalent bonds are bonds that occur during the chemical bonding of atoms of separate elements. Pairs of electrons share their energy in a way that helps to stabilize the atoms. This helps to attract and repulse atoms to one another and is a major factor in chemical reactions.
Atoms share valence electrons with each other to form chemical bonds with other atoms. This allows atoms to bond together without having to be the same element. That is, the atoms only have to be within compatible electro-negativity. When these atoms have equal electro-negativity, they can form non-polar covalent bonds.
The simplest non-polar covalent bond pairing can be illustrated with a hydrogen molecule.
H2 or H-H represents two hydrogen atoms sharing their electrons with one another in covalence. Each atom has one electron and they require at least two to be stable. When they share each other’s electrons, they fill the first electron shell.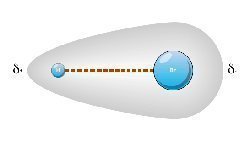
A polar covalent bond occurs between atoms that do not fully fill their outermost electron shell.
An example of this can be seen with a hydrogen chloride molecule:
H-Cl is a mixture of electrons between the Hydrogen and the Chlorine. The Hydrogen atom contains 1 electron and requires one more to fill its valence requirements. The Chlorine has a total of 17 electrons that fills the first and second shell, but requires 3 more electrons to fill its outermost shell. The total is then 18 electrons with the H-Cl molecule, which will leave the molecule positively polar charged.
A great example of non-polar covalent bonds can be seen in a methane molecule. Methane is CH4, which is one atom of carbon and 4 atoms of hydrogen. Carbon contains 6 electrons that fill the first shell and half of its second shell. It requires an additional 4 electrons to be “stable.” The 4 hydrogen atoms lend their electrons to the carbon and in turn stabilize themselves. The result is a non-polar set of 4 covalent bonds for the methane molecule. Electrons that the adjacent atoms in the bonds share are shared equally, which makes them so compatible with each other.
Essentially, no charge separation occurs when the atoms fall into place with non-polar covalent bonds. The absence of charge separation in these non-polar covalent bonds makes it impossible for interaction with water.


Comments - No Responses to “What is a Nonpolar Covalent Bond?”
Sorry but comments are closed at this time.